
Feb 20, 2024
The article highlights the discovery of a series of azetidine derivatives, known as BGAz compounds, that exhibit potent bactericidal activity against both drug-sensitive Mycobacterium tuberculosis and multidrug-resistant TB strains.

Sep 28, 2023
In preclinical research, the choice of the appropriate well system format – whether it’s a transwell, non-transwell, or individual transwell – can significantly impact the outcome of experiments. Selecting the correct plate is crucial to working with permeability, toxicity, drug screening, or other assays.

May 30, 2023
On May 15-17, the 22nd Barrier- and Transporter- days took place in Germany. At the event, Jonatan Cucala, R&D researcher at ReadyCell, presented a poster entitled “PreadyTake, an in vitro ready-to-use cell-based model to evaluate potential drug-transporter interactions.”
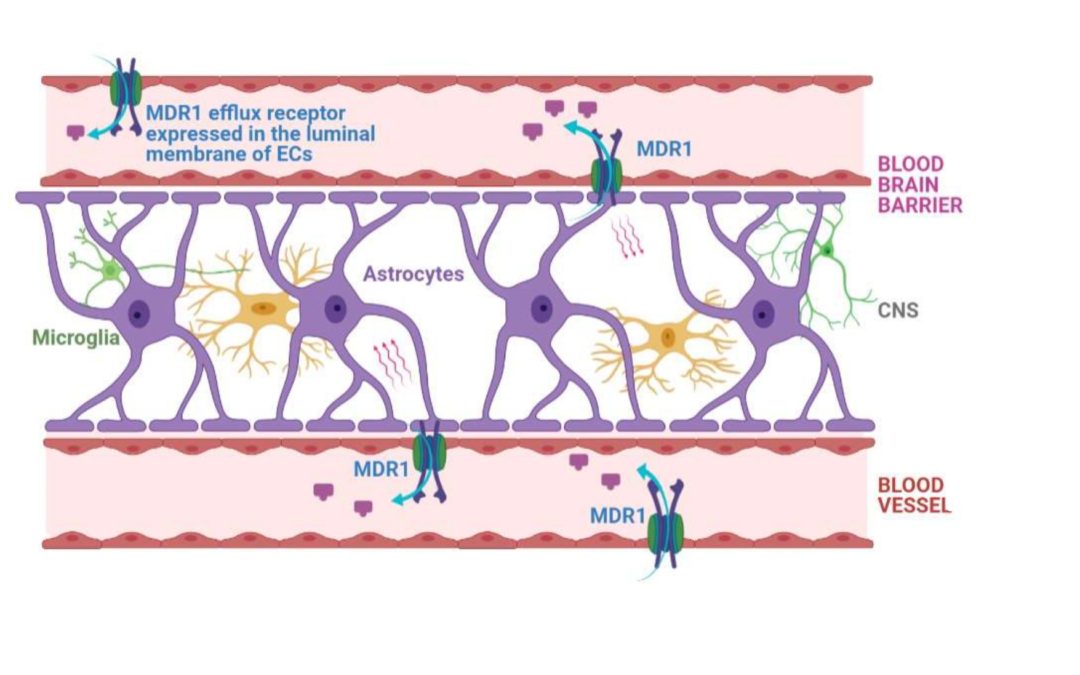
Apr 6, 2023
MDCKII cells based in vitro system is a recommended model to evaluate blood-brain barrier permeability during CNS action novel compounds.

Mar 2, 2023
Publications have confirmed the importance of developing safe chemical agents using membrane transporter analysis as a criterion. This is reflected in the field of pesticides, one of the areas in which this analysis has been most widely applied.
Sep 29, 2022
The presence of the superfamily members of the ATP binding cassette (ABC) transporters is well established as the main cause of multidrug resistance, since they efflux therapeutic compounds from cells and reduce the intracellular drug levels.







