The liver and the kidneys have clearly defined various physiologic functions, their role as excretory organs for drugs and chemicals and their polar metabolites are well described. These organs have a relevant impact on the biotransformation of a variety of chemicals and drugs.
MATE1 is one of the transporters expressed in human kidney and liver. Specifically, in the kidneys, MATE1 receptor mediates the efflux of cationic drugs and other components from renal epithelial cells into the urine. When MATE1 transporter is inhibited, a decrease of cationic drug excretion is observed, which entails a significant metabolic increase in the inner cell concentration, thus enhancing the possibility of adverse events. Researchers have agreed that MATE1 plays a vital role in cationic drug deposition and elimination, being a pivotal element to consider during novel drug preclinical stages.
In order to study possible pharmacokinetic interactions with MATE1, it is recommended to perform in vitro studies with specific cell lines that overexpress this transporter, like MDCKII or HEK293 cell lines. These in vitro assays determine the transporter-mediated transport of cationic drugs, which helps to depict a renal pharmacokinetic profile and to determine whether the sponsor should conduct in vivo studies in later stages.
ReadyCell’s research group has developed an innovative in vitro kit, Preadytake-MATE1, to evaluate possible drug-drug interactions (DDI) during renal metabolism. PreadyTake-MATE1 is a ready-to-use system for drug renal metabolism evaluation, which is based on transfected HEK293 overexpressing MATE1 transporter.
Preadytake-MATE1 has been developed to allow substrate and inhibition assessments between drugs, which can interfere in renal metabolism significantly, at a lower cost and controlled conditions.
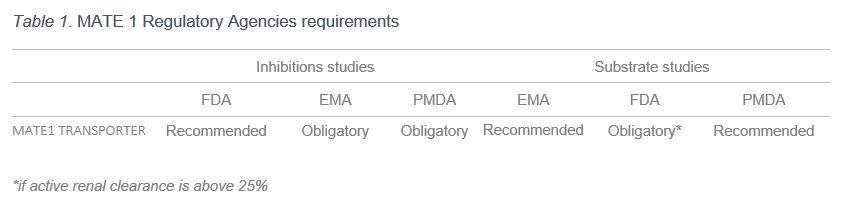
Not only the expert opinion of the International Transporter Consortium (ITC) encourages investigation of MATE1 interactions for cationic NCEs, but Regulatory Agencies also include MATE1 drug-drug interaction studies as relevant tests during preclinical trials in their guidelines.
Preadytake-MATE1 kit contains ReadyCell’s patented gelatinous Shipping Medium®. The Shipping Medium® allows optimal preservation of cultured cells at room temperature, maintaining cells in optimal conditions.
ReadyCell develops high performance in vitro kits, specially designed for ADME-Tox assays. In case you need more detailed information, contact: reagents@readycell.com





