According to the US Food and Drug Administration (FDA), a drug is considered renally eliminated when up to the 25% of the absorbed dose is excreted unchanged in the urine.
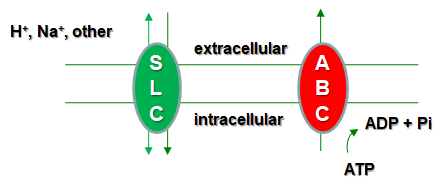
One of the major pathways for drug elimination in the kidneys is the active secretion through the proximal tubules. This is basically carried out by a series of membrane transporters belonging to the solute carrier (SLC) superfamily and the ATP-binding cassette (ABC) superfamily, both localized in the basolateral and apical membranes of the proximal tubular epithelium.
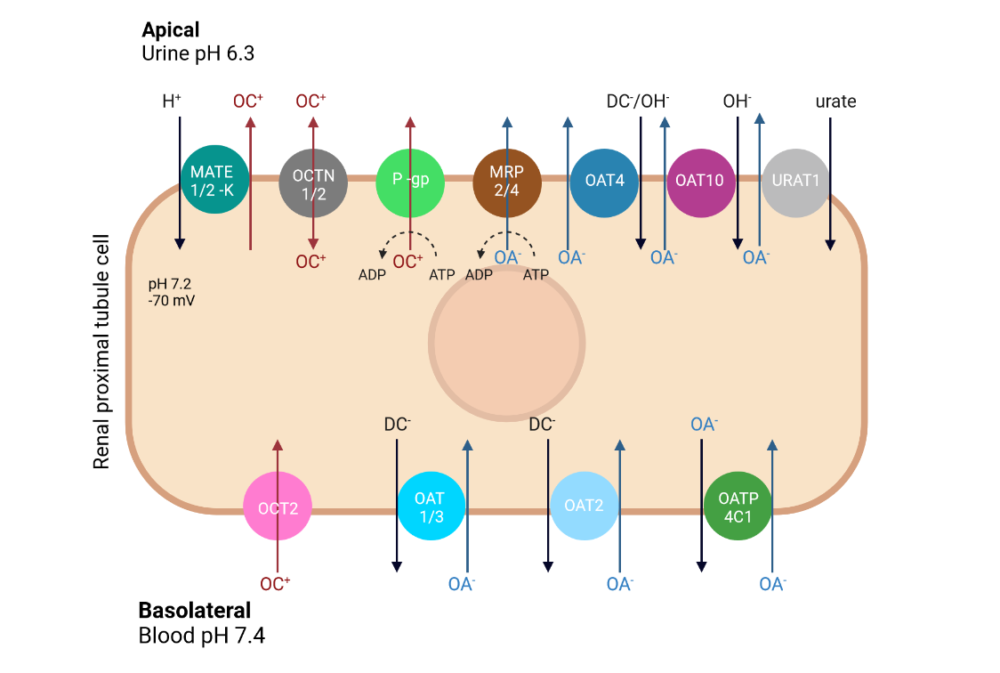
The main renal transporters in humans
- Organic cation transporters (OCTs): They include three different isoforms, being the OCT2 isoform mainly expressed in the kidney, on the basolateral membrane of the renal proximal tubule cells. In turn, OCTN1 and OCTN2 are a subfamily of the OCTs. Unlike those, OCTNs are expressed in the apical membrane of the tubule cells.
- Multidrug and toxin excretion 1 and 2 (MATE1 and MATE2): These proteins are two homologous transporters, being MATE-2K the splice variant of MATE2 in the kidney.
- P-glycoprotein (Pgp): Pgp is one of the most characterized efflux pumps. It is broadly expressed in secretory organs and tissue barriers, but also in normal tissues. In the kidney it is localized in the luminal or apical site of the proximal tubule epithelium.
- Organic anion transporters (OATs): Ten isoforms have been identified in the kidney for OATs. The most relevant, OAT1-3, OAT4 and OAT10 are localized in the basolateral and apical cell membranes, respectively. Also, eleven members of the organic anion transporting polypeptides (OATPs) have been detected. OATP4C1 is predicted to be kidney-specific.
- Multidrug resistance proteins (MRPs): The MRP2-4 is also expressed in the apical site of the tubular cells. However, the role of MRP1 and MRP3 in the kidney is unclear.
The value of renal transporter expertise in DDI clinical trials
Transporters work in tandem to eliminate drugs from the blood circulation to the urine. Although they tend to be charged selective for anionic or cationic drugs, published data suggests that there is some degree of overlap.
Understanding the role of these transporters in the clearance of new molecular entities (NME) is normally performed in-vitro at the early stages of drug development. The reason lays on the possible alteration of the pharmacokinetics/pharmacodynamics (PK/PD) of these new drugs as well as of those simultaneously administered.
Herein there are some examples of drug-drug interactions (DDIs) at the renal level, which include a rough overview of substrates and inhibitors of major human renal transporters.

For instance, OCT2 and MATE1/2K mediate metformin renal elimination. Thus, inhibition either of OCT2 which is localized at the basolateral membrane, or the inhibition of both transporters (basal and apical, respectively) will decrease drug elimination. On the contrary, inhibition of MATE1 will result in intracellular drug accumulation which could contribute to kidney injury.
However, renal transporter-mediated DDIs have also been exploited as a strategy to enhance drug concentrations or to protect the kidney. As an example, medicines which inhibit renal secretion of penicillin were concomitantly administered to prolong penicillin half-life. In contrast, coadministration of them with antivirals protect the latest of causing nephrotoxicity.
In order to ascertain in vitro whether NME are substrates or/and inhibitors of renal drug transporters, ReadyCell has available several ready-to-use cell-based assays individually overexpressing efflux and uptake transporters (e.g, MATE1, OCT2, Pgp or MDR1…). Cells are preplated and delivered worldwide by means of our patented gel-like Shipping Medium®.
For more information, contact our expert team at reagents@readycell.com