
Feb 20, 2024
The article highlights the discovery of a series of azetidine derivatives, known as BGAz compounds, that exhibit potent bactericidal activity against both drug-sensitive Mycobacterium tuberculosis and multidrug-resistant TB strains.

Sep 28, 2023
In preclinical research, the choice of the appropriate well system format – whether it’s a transwell, non-transwell, or individual transwell – can significantly impact the outcome of experiments. Selecting the correct plate is crucial to working with permeability, toxicity, drug screening, or other assays.
Jul 27, 2022
OATP1B1 and OATP1B3 are two influx transporters of the SLCO gene family that are primarily expressed in the sinusoidal membrane of hepatocytes.
Jun 28, 2022
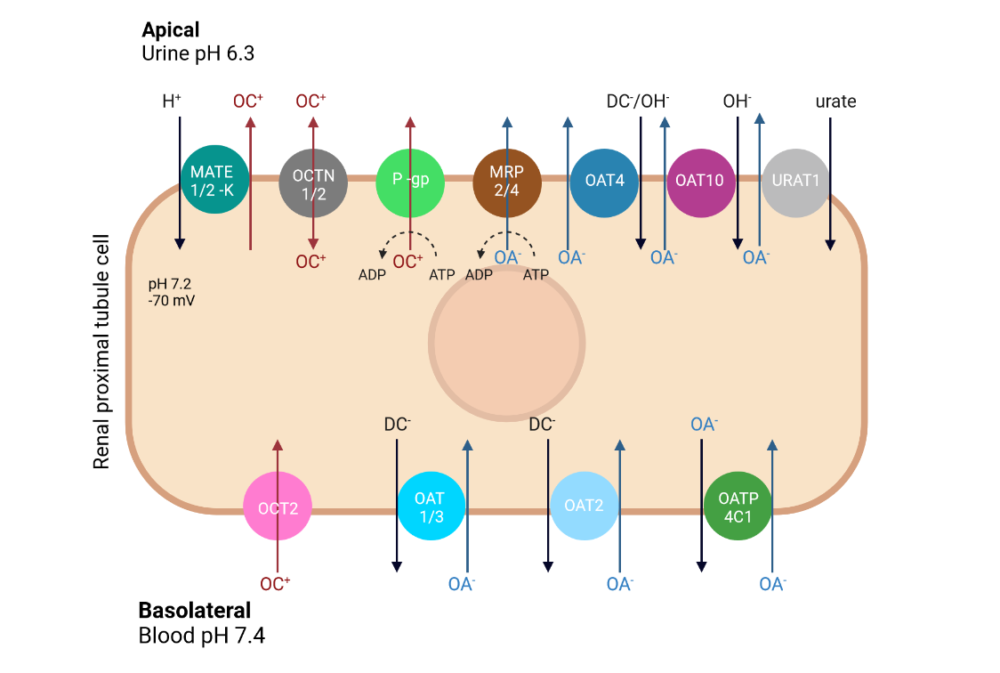
Understanding the role of these transporters in the clearance of new molecular entities (NME) is normally performed in-vitro at the early stages of drug development.

Apr 21, 2022
It is well known that food intake changes luminal conditions (e.g. pH, motility, microbiota,…) in the stomach and the small intestine, modifying drugs bioavailability. Food-drug interactions are one of the major challenges for oral-administered drugs, even more so if considering the growing use of food supplements and functional foods.






