Last July an investigation led by the researcher Nicoló Milani was published in the prestigious analytical journal Lab on a Chip. Under the title Application of a gut-liver-on-a-chip device and mechanistic modelling to the quantitative in vitro pharmacokinetic study of mycophenolate mofetil, the CacoGoblet kit was employed to investigate the permeability and the metabolism of the compound in a gut-liver-organ-on-a-chip (OoC).
Organ-on-a-chip, a continuously evolving model
Current models of pre-clinical drug discovery and development, as well as commonly used animal models, are often not suitable for the prediction of human pharmacokinetics (PK). Therefore, one of the biggest challenges today in the field is to find an effective predictive model.
In this way, microphysiological systems (MPS) consisting of multiple linked OoC components are promising tools that provide relevant in vitro to in vivo translation of drug disposition, efficacy and toxicity.
Roche’s study tested mycophenolate mofetil and its metabolites for quantitative evaluation and employed a combination of HT-29 and Caco-2 cells with intestinal-liver OoC.
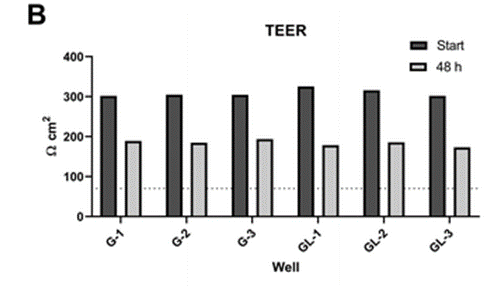
Experimental results
According to the study, the gut-liver OoC system allowed to study the pharmacokinetics of mycophenolate mofetil and its two main metabolites. This opened up the possibility for further research of prodrugs where a combination of intestinal and hepatic processes determine the drug and metabolite exposure.
Furthermore, the team defined a robust and rigorous in silico modelling strategy which was fundamental for the evaluation of the data from the combined gut-liver system.
Finally, the demonstrated ability of the gut-liver OoC to capture the intestinal and liver metabolism of mycophenolate mofetil supports its potential for prediction of pharmacokinetics and drug-drug interaction risk of other orally administered drugs with complex in vivo behavior.
Read full article: Application of a gut–liver-on-a-chip device and mechanistic modelling to the quantitative in vitro pharmacokinetic study of mycophenolate mofetil