Drug-drug interactions (DDI) are an important risk factor for polymedicated patients, as they can compromise safety and alter the efficacy of medical treatments. These interactions are usually related to drugs, however, they have also been identified between nutritional products and drugs. Hence the need to prioritize the assessment of pharmacokinetic relevant molecules, cytoplasmatic enzymes, uptake transporters, and efflux receptors. When a new drug is in development phases, it is crucial to understand metabolic interactions and optimize the compound’s therapeutic effect. The scientific community concludes that relevant pharmacokinetic DDI can occur via different mechanisms.
- alterations in drug absorption
In oral dosage, drugs solubility and dissolution change as a consequence of altered gastric homeostasis, which affects several parameters, such as pH and time of exposure.
- protein-binding sites displacement
Drugs also interact ambivalently with alternative compounds, present in plasma or other biologic liquids, which affect or prevent affinity to a target molecule.
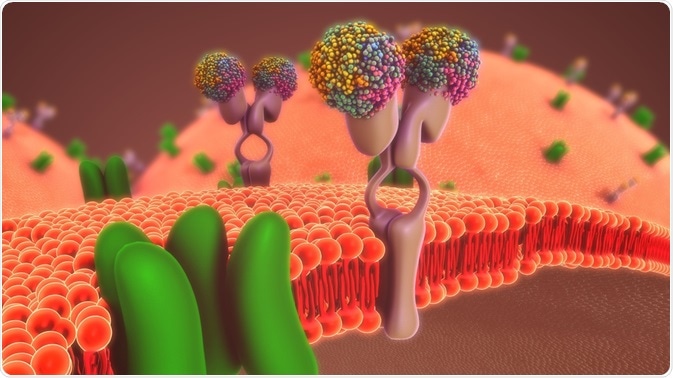
- interactions with transmembrane PROTEIN transporters
Inhibition of efflux and uptake transporters may lead to alterations in the molecules’ transfer between inner and outer cell spaces.
- Cytoplasmatic enzymes alteration
When a studied xenobiotic strongly interacts with relevant pharmacokinetic enzymes, mostly CYP450 isoforms such as CYP3A4, it can impact organ metabolic pathways. Regulatory experts recommend the use of Caco-2 cells and genetically modified MDCKII and HEK293 cells at preclinical stages, to generate preliminary data related to DDI. Further knowledge of xenobiotic interactions with pharmacokinetic relevant transmembrane proteins, such as MDR1 (Pgp), BCRP, MATE1, MRP2, and OCT2 will facilitate posterior clinical phases and will prevent potential adverse events. ReadyCell is specialized in cell-based in vitro kits developed for scientific research, which extrapolates in vivo conditions in a ready-to-use product. In case you require additional information about cell-based kits to assess drug-drug interactions, contact our scientists at reagents@readycell.com. Additional readings: Kathleen M Giacomini et al. Membrane transporters in drug development, 2010. Nat Rev Drug Discov 9:215-36 Xinfeng Zhang et al. Intestinal absorption mechanisms of berberine, palmatine, jateorhizine, and coptisine: involvement of P-glycoprotein, 2011. Xenobiotica 41:290-6 Xiaodong Liu. Transporter-Mediated Drug-Drug Interactions and Their Significance, 2019. Adv Exp Med Biol 1141:241-291