Toxicological analysis of xenobiotics or chemicals intended for routine tasks, such as products used for hygiene, household cleaning, perfumes, environmental compounds, agricultural or industrial production is essential to ensure the proper formulation of the final product in terms of efficacy and safety.
One of the key parameters is the concentration of the chemical compound, since an inadequate dose can affect standard human metabolism and increase the possibility of adverse effects. Some studies have confirmed that these chemical molecules at specific concentrations can particularly modulate the activity of the membrane transporters P-GP and BCRP.
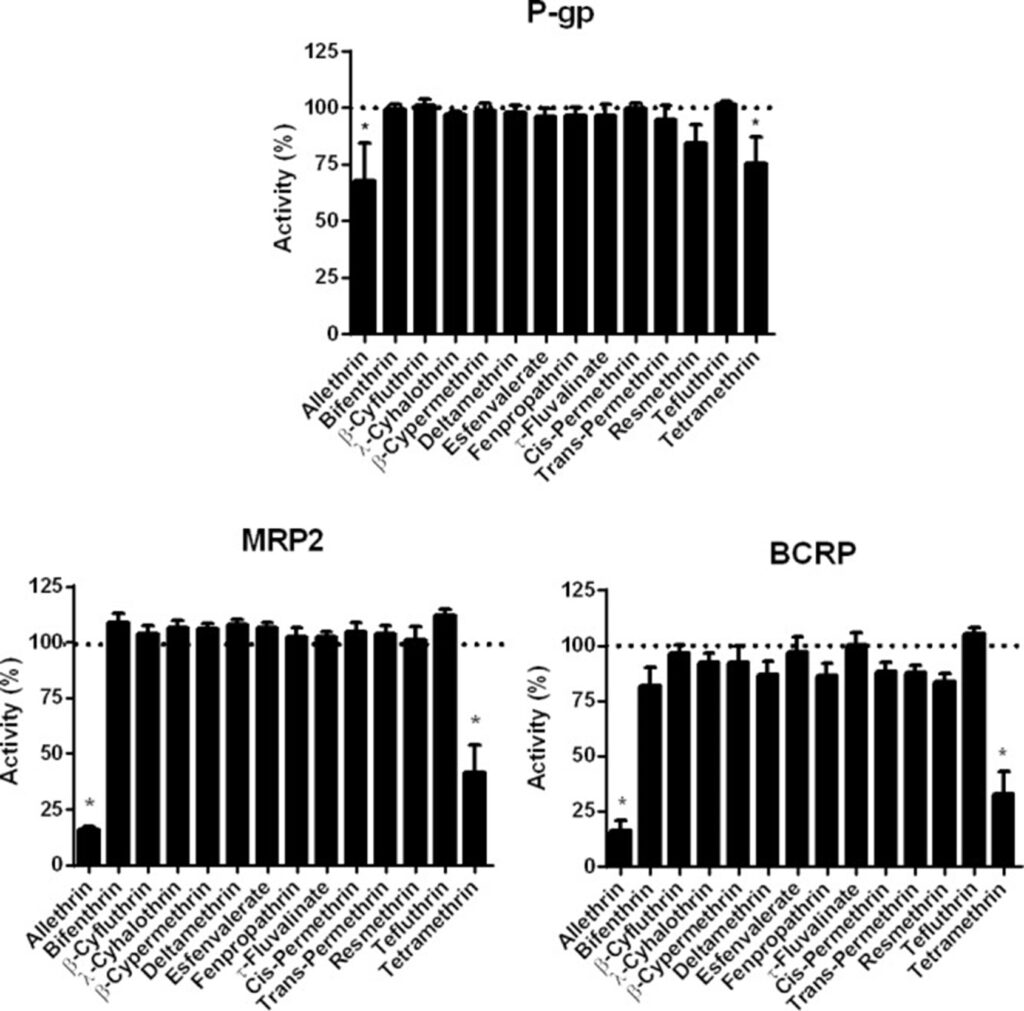
Efflux transporter examples in the determination of toxicity results
Membrane or efflux transporters such as BCRP and MDR1 (P-gp) play an important role in regulating the kinetics of xenobiotics, whose primary function is to metabolize compounds in inner tissues. In pharmacology projects, transporters are studied to evaluate molecule metabolism and identify non-desired interactions that affect transport between tissues, especially during preclinical phases.
Publications have confirmed the importance of developing safe chemical agents using membrane transporter analysis as a criterion. This is reflected in the field of pesticides, one of the areas in which this analysis has been most widely applied.
One example of this are the pyrethoids, insecticides that humans may be exposed to. A 2017 study led by Lisa Chedik revealed that they inhibit BCRP in the nM range while P-gp also showed a moderately inhibition.
On the other hand, in 2020, Le Vée studied the interaction of Neonicotinoid pesticides with drug pharmacologically relevant receptors: acetamiprid, clothianidin, imidacloprid, nitenpyram, thiacloprid and thiacloprid amide, at a specific concentration of 100µM. No interaction with MDR1 nor with BCRP was detected.
These studies opened a debate to control the use of specific chemicals and served to define the appropriate group of pesticides based on intended use and human safety.
Caco-2 and MDCKII, the most commonly used cell lines for in vitro toxicological studies
As we have seen, efflux transporters play a crucial role in metabolism, and therefore, it is recommended to study the interaction of molecules with these transmembrane transporters, P-gp and BCRP, to understand and characterize the safety profile of a specific compound and define its adequate use based on the intended use.
The main advantage of in vitro testing is the possibility to obtain initial data applying advanced biotechnology with reduced efforts. In addition, in vitro assays with Caco-2 and MDCKII cells have demonstrated being an adequate tool to study the non-desired interactions related to pharmacological transporters:
- Caco2 cells: Specific cell lines that express the efflux transporters MDR1 and BCRP. It is possible to evaluate P-gp and BCRP interaction adding reference substrates such as Digoxin and Prazosin and testing the efflux ratio in qualitative terms with the studied compound.
- Transfected MDCKII cells: These cell lines overexpress BCRP and/or MDR1 transporters and allow to specify the kinetics related to pharmacological relevant receptors. When the compound under study interacts with the transporter, a decrease in active transport should be detected.
In vitro toxicological studies are required to understand the safety profile of a compound with medical, environmental or even technological indication and define the adequate use.
At ReadyCell we have in vitro models indicated for cell toxicity research, as well as an expert team that will solve all your doubts regarding the characterization of compounds in development phases.
For more information, contact our expert team at reagents@medtechbcn.com
Additional readings
- Le Vée, M, Bacle, A, Bruyere, A, Fardel, O. Neonicotinoid pesticides poorly interact with human drug transporters. J Biochem Mol Toxicol. 2019; 33:e22379. https://doi.org/10.1002/jbt.22379
- Guéniche, N., Bruyere, A., Le Vée, M. and Fardel, O. (2020), Implication of human drug transporters to toxicokinetics and toxicity of pesticides. Pest. Manag. Sci., 76: 18-25. https://doi.org/10.1002/ps.5577
- Chedik L, Bruyere A, Le Vee M, Stieger B, Denizot C, Parmentier Y, Potin S, Fardel O. Inhibition of Human Drug Transporter Activities by the Pyrethroid Pesticides Allethrin and Tetramethrin. PLoS One. 2017 Jan 18;12(1):e0169480. doi: 10.1371/journal.pone.0169480.





