READYCELL KITS APPLICATIONS
In Vitro Cytotoxicity Assays
Identifying potential toxicity at early research stages allows to optimize new compounds development.
ReadyCell CYTOTOXICITY ASSAY KITS
Advancing cell toxicity evaluation with ready-to-use cell-based plates.

Say goodbye to outdated colorimetric assays!
Our new application notes on CacoReady plates will show you how to accurately predict and stratify intestinal compound toxicity evaluating the TEER value and the LY paracellular flux, two relevant indicators of the cell barrier integrity.
Get for free the latest protocol for acute toxicity assessment.
CELL TOXICITY ASSAY
Ensure the safety of your new molecule
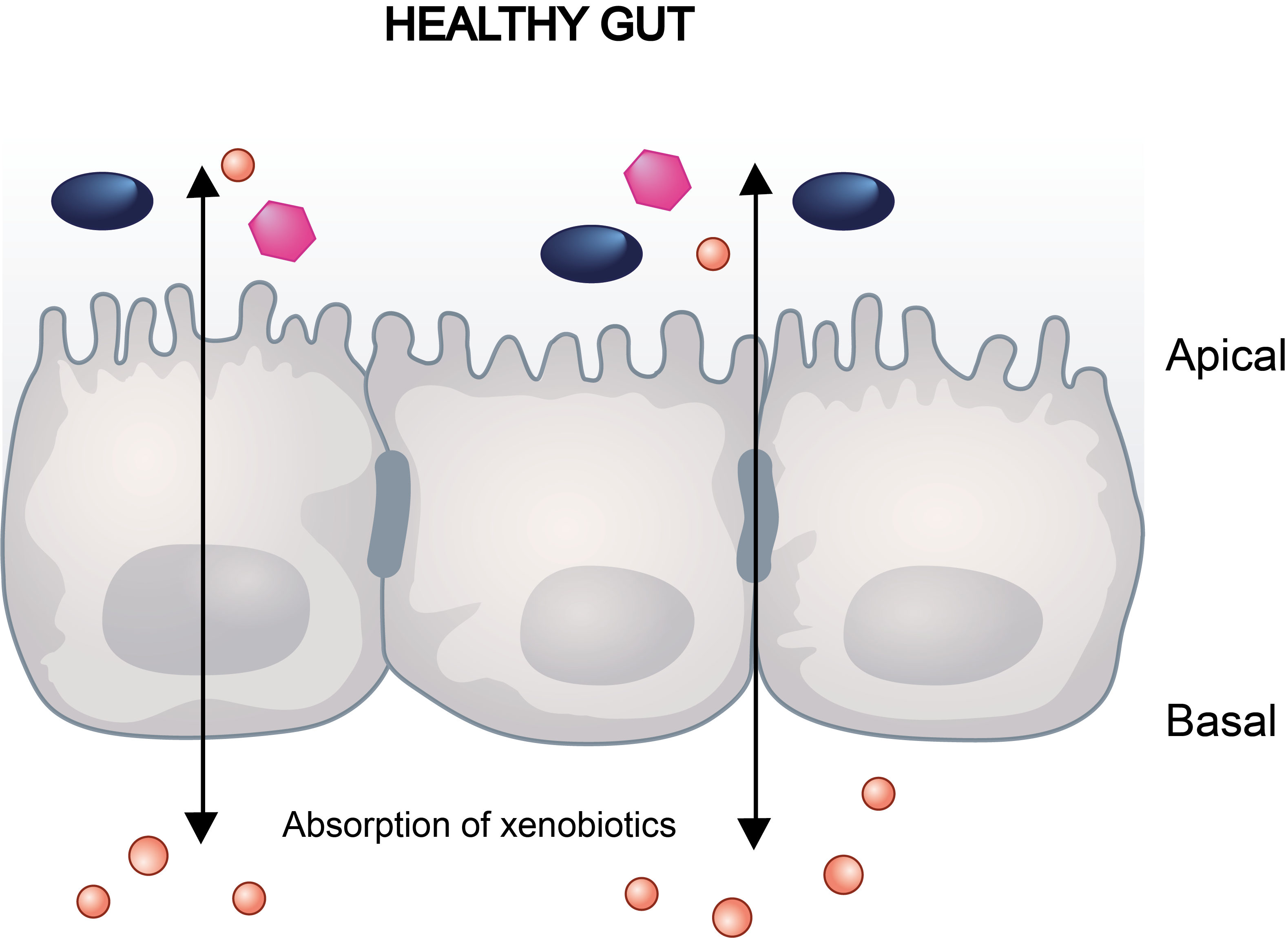
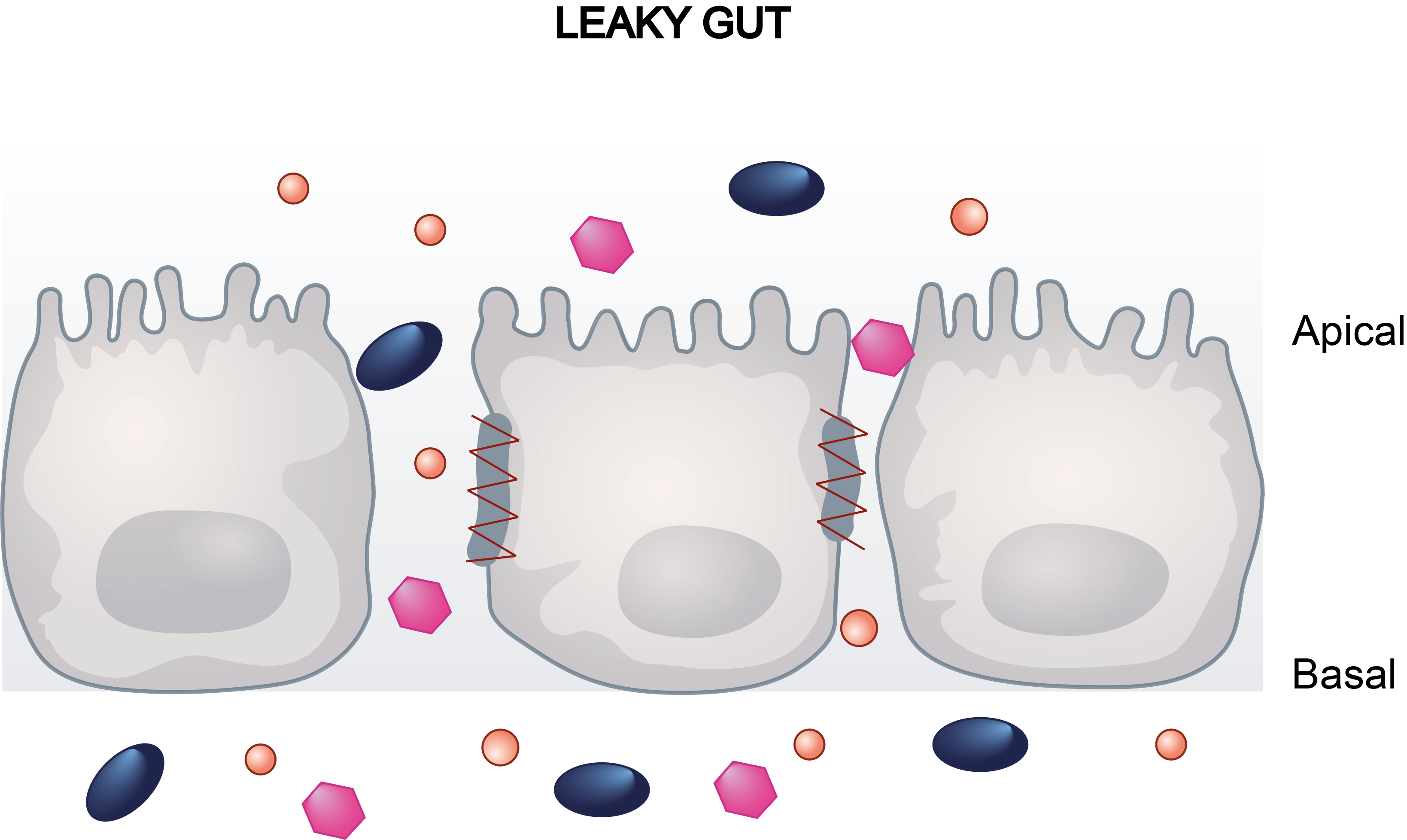
Drug-induced gastrointestinal reactions are one of the main causes of post-market drug withdrawal. These adverse effects can potentially be predicted by in vitro assays, since these side effects, like diarrhea, correlate with intestinal barrier dysfunction. In this regard, the most commonly used models for intestinal drug studies in pharmaceutical research consist of human intestinal epithelial cells, such as the colon adenocarcinoma cell line Caco-2.
There is a growing interest in using in vitro cytotoxicity tests instead of in vivo test systems to assess the cell viability of new molecular entities. This approach offers several advantages like reducing the need for animal testing, lowering costs, and speeding up the development and availability of these molecules. Although we should be cautious when comparing results from in vitro and in vivo tests due to their complexity, it is often observed that short-term cytotoxicity tests show positive responses that align well with short-term in vivo implantation tests.
CYTOTOXICITY ASSAY PROTOCOL
Cell toxicity testing and stratification in transwell plates
READYCELL Support
Frequently Asked Questions
What are the differences between transwell, no-transwell and individual transwell?
Caco-2 cells seeded on transwell inserts allow them to differentiate and polarise with an apical and basal surface which is mandatory to establish their barrier function. The Transwell system let using cell barrier disruption as an indicator of compounds’ toxicity. Transwells can be either integrated or singles, the latter recommended for assays requiring individual mobility at different time periods or measurements.
The non-Transwell format contains differentiated but not polarised Caco-2 cells. Late-stage indicators through colourimetric tests are used to measure the adverse effects of tested compounds.
For an effective cytotoxicity assay protocol, as well as a permeability assay protocol, it’s crucial to consider the choice of well system carefully.
What do the current regulations establish on the basis of cell toxicity tests?
Which are the main reference compounds for CacoReady and what are the differences between them?
Is it possible to add a testing service to the order?
Which reagents are suitable for cell viability assays using non-transwell plates?
Is the quality control data specific for each kit?
Does ReadyCell shipping medium affect cell lines?
No, ReadyCell’s Shipping Medium consists of a semi-solid culture system specifically designed to preserve cells at room temperature (15-25ºC). This medium maintains a suitable physicochemical environment, keeping adequate moisture conditions for cellular homeostasis and forming a protective cushion that protects cell integrity and functionality during long-distance shipments up to seven days.
Related news
Recommended reference compounds for permeability assays
Permeability assays are important for understanding how molecules cross biological barriers, assessing both their ability to cross and the mechanisms involved. While drugs are often absorbed by passive diffusion, some molecules can also be substrates for membrane proteins or transporters.
Caco-2 Permeability Assay Protocol
The permeability assay protocol for Caco-2 cells serves as a cornerstone in understanding and predicting the intestinal absorption of pharmaceutical agents.
A pioneering investigation unveils novel allosteric inhibitors of SHP2 in cancer therapy
In a recent publication in the Journal of Medicinal Chemistry, a collaborative research led by the scientist Dr. H. Day, in conjunction with a consortium of researchers, explored the development of allosteric inhibitors targeting SH2 domain-containing protein tyrosine phosphatase-2 (SHP2) for cancer therapy.






