Hepatitis C virus (HCV) is a hepatic disease caused by an RNA virus that can lead to hepatic cirrhosis and hepatocellular carcinoma. The use of non-sterile medical procedures is the most common risk factor for HCV infection.
Until there is a prophylactic vaccine to prevent HCV, the infected population is treated with interferon and ribavirin, which respond favorably to about 50% of patients. For those unresponsive to IFN-based therapies, HCV is treated with a combination of new direct-acting antiviral agents (DAAs). Although, the combination of these drugs has been found to be highly effective, above an 80% rate, preclinical studies have identified significant risks of drug-drug interactions (DDIs) especially for those patients with higher number of co-medication. Inhibition of the organic anion transporting polypeptides (OATPs) and the multidrug resistance protein 2 (MRP2) are some examples of DAAs interactions.
How do transporters act in liver drug/endogenous substrate elimination?
OATP1B1 and OATP1B3 are two influx transporters of the SLCO gene family that are primarily expressed in the sinusoidal membrane of hepatocytes. They both play a key role in the pharmacokinetics of various drugs as they are involved in the uptake of a broad range of xenobiotics, drugs and substrates. For instance, impaired elimination of unconjugated bilirubin by DAAs is the result of a potent inhibition towards OATP1B1/1B3 and a mild inhibition on MRP2, a membrane transporter localized in the hepatocyte canalicular membrane. Consequently, there is an increase in plasma bilirubin, a clinically relevant adverse event named hyperbilirubinemia.
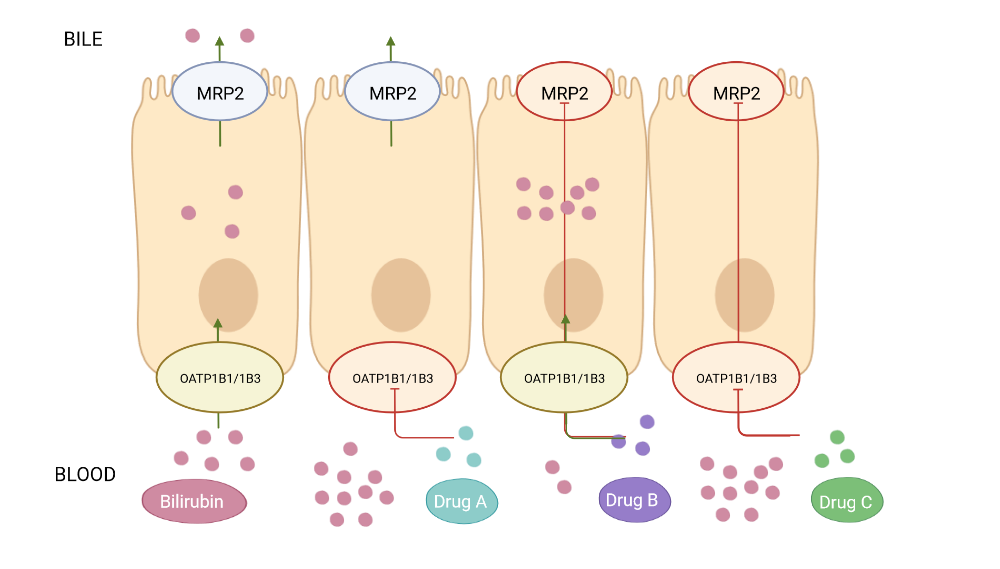
Diagram of bilirubin pathway in Madin-Darby Kidney Cells type II (MDCKII) overexpressing MRP2 and OATP1B1 and OATP1B3 transporters. Bilirubin, Drug A: OATP1B1/1B3 inhibitor, Drug B: MRP2 inhibitor and Drug C: MRP2 and OATP1B1/1B3 inhibitor.
Prediction of hepatic drug clearance
To identify drugs that may induce hyperbilirubinemia it is recommended to monitor drug interaction with OATP1B1/B3 and MRP2 transporters. Fluorescent probes such as pyranine, Cascade Blue hydrazide (CB) and sulforhodamine 101 (SR101) have been used to rapidly characterize uptake and efflux transporters in Madin-Darby Kidney cells type II (MDCKII) overexpressing these membrane proteins. Although fluorescent dyes are probably non-specific and could be substrates of other hepatocyte transporters, the data obtained in the fluorescent-based assays can be applied to evaluate DDI potential drug candidates. Combining in vitro data and clinical outcomes will strengthen the predictive power of these trials.
To ascertain whether a drug’s hepatic uptake into the liver exert a pharmacological effect, the US Food and Drug Administration (FDA) recommends conducting uptake in vitro assays in OATP1B1/1B3 transfected cells. According to the guidelines, an investigational drug will be considered a substrate of these transporters when drug uptake will be twice than the cells expressing the vehicle or an inhibitor when it will decrease drug uptake by 50%.
For in vitro prediction studies of substrates or/and inhibitors of hepatic transporters, ReadyCell has available several ready-to-use cell-based assays individually overexpressing uptake and efflux transporters (e.g, OATP1B1, OATP1B3 and MRP2.). Cells are preplated and delivered worldwide by means of our patented gel-like Shipping Medium®.
For more information, contact our expert team at reagents@medtechbcn.com
References
- Manns MP et al. Nat rev Dis Primers 2017, 3:17006 (doi: 10.1038/nrdp.2017.6)
- Furihata T et al. Antimicrob Agents Chemother 2014, 58:4555 (doi: 10.1128/AAC.02724-14)
- Binda C et al. European Rev Me Pharmacol Sci 2017, 21 (1 Suppl):102
- Székely V et al. European J of Pharma Sci 2020, 151 (https://doi.org/10.1016/j.ejps.2020.105395)
- Tátrai P and Krajcsi P. Pharmaceutics 2020, 12:755 (https://doi.org/10.3390/pharmaceutics12080755)
- Guidance for Industry (fda.gov). January 2020 (https://www.fda.gov/media/134582/download)