| Programme | CIEN 2019 (CDTI) |
| Budget | 505.321 Euros |
| Duration | 2019-2023 (48 months) |
| Consortium | Grupo Uvesa (project leader), Idukern, Betelgeux, Incarlopsa, Biolan, Delafruit and ReadyCell |
As part of the collaborative consortium SEGURAM, our focus lied within the realm of innovative strategies aimed at enhancing productivity, quality, and safety within the meat industry, while concurrently reducing reliance on antibiotics.
Development of a gut-on-a-chip
ReadyCell has designed and developed a microfluidic gut-on-a-chip device that mimics the key properties of the intestine to study nutrient bioavailability and product safety, aligning with the goals of the SEGURAM project framework.
The prototype, based on PMMA (polymethyl methacrylate), integrates two independent channels separated by a PET membrane. In the upper channel, Caco-2 cells are cultured to form a monolayer on the membrane, while the lower channel is used to introduce culture medium and collect basal fluid. The prototype, optimized with 5 subunits per device and standardized size, is compatible with commonly used microscope slides (76×26 mm) and therefore, with various laboratory equipment. Additionally, the high transparency of the device allows for microscopic assays to be conducted without manipulation and disassembly.
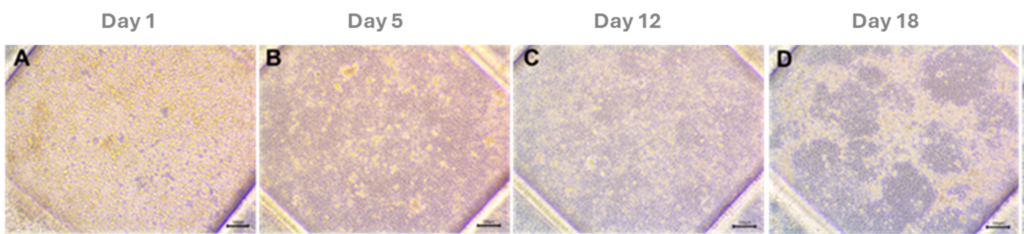
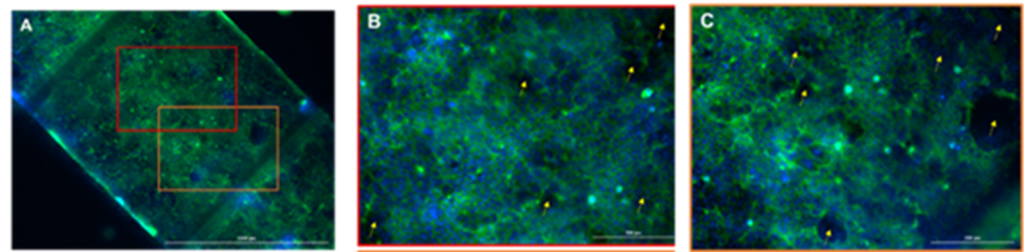
The evaluation of cell growth and epithelial barrier formation using microscopy techniques highlighted the adhesion and formation of a cell monolayer on the PET membrane. Lucifer Yellow (LY) permeability assay was conducted to assess barrier integrity. Results confirmed that Caco-2 cells successfully formed a well-developed epithelial barrier by the fifth day of culture, establishing this day as optimal for conducting ingredient bioavailability assays. The acceptance criteria set for this gut-on-a-chip model were Papp < 10·10-6 cm/s and flow < 0.5%.
Concurrently, efforts included adapting ReadyCell’s Shipping Medium, originally designed for transporting our Caco-2 models in 2D plate formats, for using in the new organ-on-a-chip models and aiming to transport prototypes at room temperature.
Bioavailability assays in gut-on-a-chip
ReadyCell aimed to validate gut-on-a-chip devices’ use in nutrient bioavailability research. Attempts were made to correlate in vitro nutrient bioavailability assay results using feed extracts from in vivo studies with analysis of samples collected (feed and feces) from poultry farm studies. An acid-free extraction methodology was implemented to analyze nutrients and ginger components in animal feed samples provided by some consortium partners, confirming minerals and gingerol detection studies without compromising the integrity of the intestinal barrier.
Difficulties in detecting the components of interest through analytical techniques in the in vitro samples hindered correlating the mineral absorption and gingerol impact observed in vitro with the in vivo patterns. Despite these analytical challenges, experimental procedures for gut-on-a-chip bioavailability assays were developed and optimized to minimize variability sources. Gut-on-a-chip device selection streamlined in vivo intestinal environment simulation compared to 2D plate systems and proved useful for bioavailability and efficacy assays. Yet, continued research is essential to enhance biological relevance and validate the system.
Results


Companies












