READYCELL KITS APPLICATIONS
In Vitro Permeability Assay
The use of our products manufactured with Caco-2, HT-29 and MDCKII cells for permeability assays will help you to investigate your compound absorption across a relevant barrier.
CELL PERMEABILITY ASSAY MODELS
Ready-to-use cells for preclinical stages
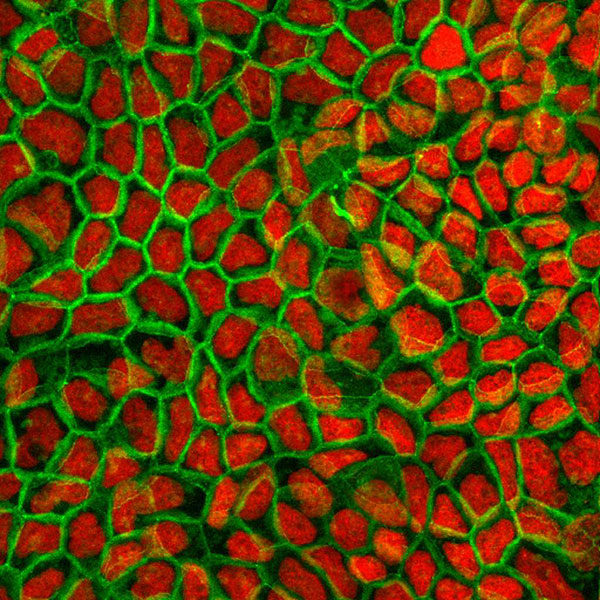
Caco-2 permeability assay
Read more
Caco-2 cells are considered an in vitro gold standard for assessing oral drug absorption. This model provides reliable predictive permeability data either by passive diffusion and/or active transport for hydrophilic to moderately lipophilic compounds and can easily be adapted for high-throughput screening purposes.
The Caco-2 model is also useful to assay active transport of drugs in the central nervous system (CNS) since it expresses two efflux transporters that play a relevant role in the permeation of compounds across the blood brain barrier (BBB).
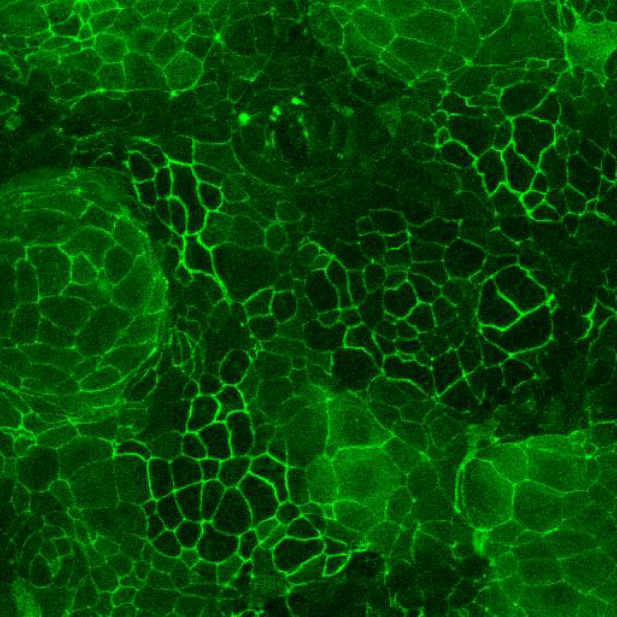
Caco-2 and HT-29 permeabilty assay
Read more
CacoGoblet is also considered a relevant cellular model for studies of drug absorption across the gastrointestinal barrier, either by passive diffusion and/or by active transport. The model can be easily adapted for high-throughput screening purposes. In addition to drug permeability testing CacoGoblet respond well to inflammatory mediators, providing valuable information on the efficacy of anti-inflammatory drugs.
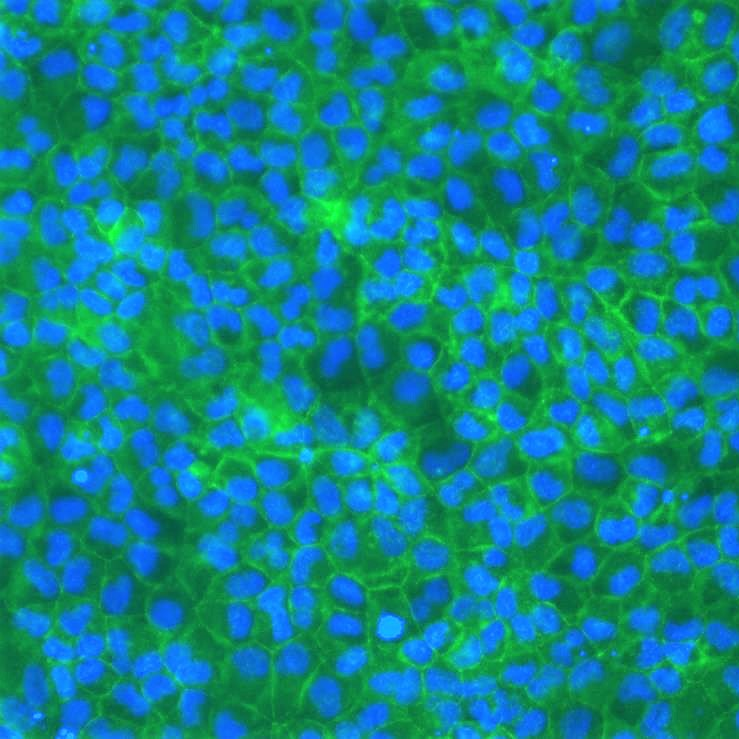
MDCKII permeability assay
Read more
In addition, MDCKII cells are also efficient for transient and stable gene transfection, making them especially relevant to overexpress uptake and efflux drug transporters such as two members of the ATP-binding Cassette (ABC) superfamily, MDR1 and BCRP.
ReadyCell permeability kits
We help you to determine your compound suitability for oral dosing
Studying the permeability of compounds across a cell monolayer is a well-established in vitro technique to screen for oral absorption and evaluate drug transporters.
Our range of pre-plated transwell products allows the rapid and accurate determination of drug transport across Caco-2, HT-29 and MDCKII cell monolayers.
ABSORTION ASSAY PROTOCOL
Reference values for Caco-2 permeability assay
If you want a comprehensive guide on conducting a Caco-2 permeability test procedure, check out this detailed protocol.
READYCELL Support
Frequently Asked Questions
Is the quality control data specific for each kit?
What is the difference between CacoReady and CacoGoblet?
Because they consist of different cell phenotypes, the barrier properties are also different being very tight for CacoReady with TEER values ≥ 1000 ohms x cm2 and looser for CacoGoblet (≥ 70 ohms x cm2), being the drug permeability assays for both models very consistent.
Does ReadyCell shipping medium affect permeability cell lines?
Is it possible to add a testing service to the order?
What are the differences between transwell, no-transwell and individual transwell?
For an effective permeability assay protocol, as well as a cytotoxicity assay protocol, it’s crucial to consider the choice of well system carefully.
What do the current regulations establish on the basis of permeability tests?
Which are the main reference compounds for CacoReady and what are the differences between them?
Related news
Recommended reference compounds for permeability assays
Permeability assays are important for understanding how molecules cross biological barriers, assessing both their ability to cross and the mechanisms involved. While drugs are often absorbed by passive diffusion, some molecules can also be substrates for membrane proteins or transporters.
Caco-2 Permeability Assay Protocol
The permeability assay protocol for Caco-2 cells serves as a cornerstone in understanding and predicting the intestinal absorption of pharmaceutical agents.
A pioneering investigation unveils novel allosteric inhibitors of SHP2 in cancer therapy
In a recent publication in the Journal of Medicinal Chemistry, a collaborative research led by the scientist Dr. H. Day, in conjunction with a consortium of researchers, explored the development of allosteric inhibitors targeting SH2 domain-containing protein tyrosine phosphatase-2 (SHP2) for cancer therapy.








