ReadyCell News
Stay up-to-date on the latest ReadyCell news, projects and developments.
The evaluation of drug interactions in preclinical stages optimizes novel drug development
Specialized manufacturer of in vitro kits indicated to evaluate pharmacological relevant efflux and uptake receptors for TDI assays.
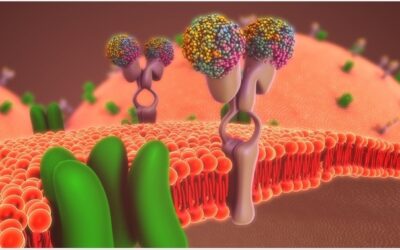
Role of pharmacological efflux receptors in cancer treatment resistance
Transmembrane efflux receptors, Pgp, MRP1 and BCRP has shown a relevant effect in the chemoterapy resistance.
Recommended cell-based in vitro testing to assess BCRP protein-mediated transport
PreadyPort BCRP is a in vitro system based on transfected MDCKII cells overexpressing the BCRP transporter.
3rd SCI-RSC Symposium on Transporters in Drug Discovery and Development
ReadyCell scientists shared their expertise ane experience on the impact of pharmacologically relevant drug-drug interactions.
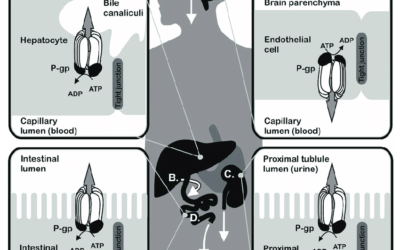
MDCK II cells overexpressing MDR1 protein. A must assay for drug development.
ReadyCell provides ready-to-use in vitro system for assessing the pharmacological role of the P-gp receptor in preclinical stages.
Pharmacology 2020 Event
ReadyCell actively interacts with the Drug Discovery community, introducing cell-based in vitro systems and easing novel compounds’ preclinical development.
Discover how in vitro Caco-2 cells help you evaluate novel therapeutic molecules
Caco-2 cells are considered a reference cellular model for basic research and drug discovery projects. ReadyCell supplies systems worldwide.
Readycell’s gratitude message
Due to the current situation and special circumstances, ReadyCell’s team would like to thank all healthcare professionals for the huge effort to contain the pandemic, which allows to carry on our daily lives.
PreadyTake MATE1, Screening Renal and Hepatic Drug Metabolism
In vitro systems based on HEK293 cells are a recommended technique to assess MATE1 transporter interactions during preclinical stages.
The latest FDA guidance on in vitro drug-drug interactions is finally out!
Among other things, the agency explains that DDIs are a critical factor in a drug’s overall benefit-risk profile.
Meet us at BioAsia 2020 in India
ReadyCell is happy to announce that its team will be attending this year’s BioAsia Conference taking place from 17 – 19 February 2020 in Hyderabad, India.
ReadyCell at “The In Vitro Choice” Meeting
The speakers list included basic researchers and companies responsibles in the field of safety and toxicity assays.